Scalp Psoriasis

SORILUX Foam delivers effective, steroid-free treatment for scalp psoriasis
SORILUX Foam was evaluated as monotherapy for treatment of moderate plaque psoriasis of the scalp in a multicenter, randomized, double-blind, vehicle-controlled study in 363 patients aged 12 to 97 years (11 patients were <18 years). 1
Study design 2
- At baseline, patients were required to have ≥10% involvement of the total scalp surface area with an Investigator’s Static Global Assessment (ISGA) score of 3 (moderate) on a 6-point scale for erythema, thickness, and scaliness
- Patients were instructed to self-administer SORILUX Foam or vehicle foam as monotherapy twice daily, morning and evening, for 8 weeks
Treatment success was defined as an ISGA score of 0 (clear) or 1 (almost clear) at week 8. 3
Think you know calcipotriene? Look again.
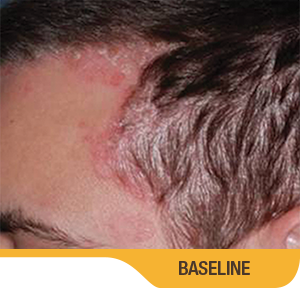
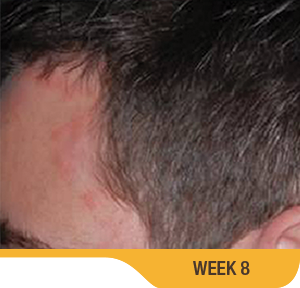
SORILUX Foam—photos from clinical trials
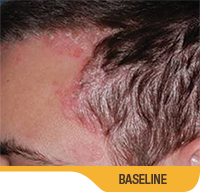
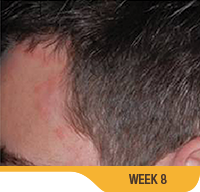
Actual patient from clinical trials with moderate plaque psoriasis (ISGA score 3) at baseline, representative of patients who achieved treatment success. Individual results may vary.
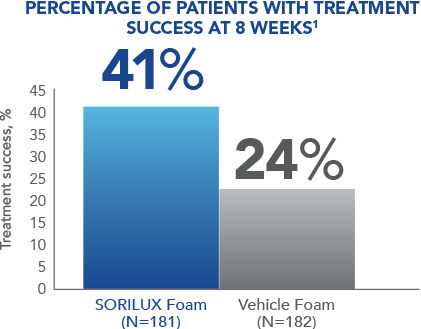
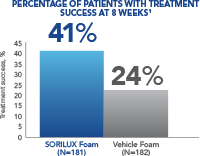
In a Phase III clinical study of moderate-severity scalp psoriasis, SORILUX Foam monotherapy achieved significant results when compared with vehicle foam 1
At week 8, 41% of patients treated with SORILUX Foam achieved treatment success compared with 24% of patients treated with vehicle foam ( P<0.001). 1
SORILUX Foam was well tolerated. The rate of adverse events was similar to that of vehicle foam.
APPLICATION SITE REACTIONS FROM THE PIVOTAL STUDY FOR PLAQUE PSORIASIS OF THE SCALP 2
| ADVERSE EVENTS REPORTED IN >2% OF PATIENTS | SORILUX FOAM (N=181) |
VEHICLE FOAM (N=182) |
|---|---|---|
| Application site pain | 4% | 3% |
| Application site pruritus | 4% | 4% |
| Application site erythema | 3% | 0% |
References: 1. Feldman SR, Eastman WJ, Brundage T, Mills M. A multicenter, randomized, double-blind study of the efficacy and safety of calcipotriene foam, 0.005%, vs vehicle foam in the treatment of plaque-type psoriasis of the scalp. J Drugs Dermatol. 2013;12(3):300-306. 2. Data on file. Greenville, NC; Mayne Pharma LLC. 3. SORILUX Foam [package insert]. Greenville, NC: Mayne Pharma; 2019.